Additional Flexibility For Veterinarians As CYTOPOINT® Use Extended To Cover The Treatment Of Pruritus Associated With Allergic Dermatitis In Dogs
Zoetis’ antibody therapy, CYTOPOINT®, already approved in the EU for the treatment of clinical manifestations of atopic dermatitis in dogs now has wider application.
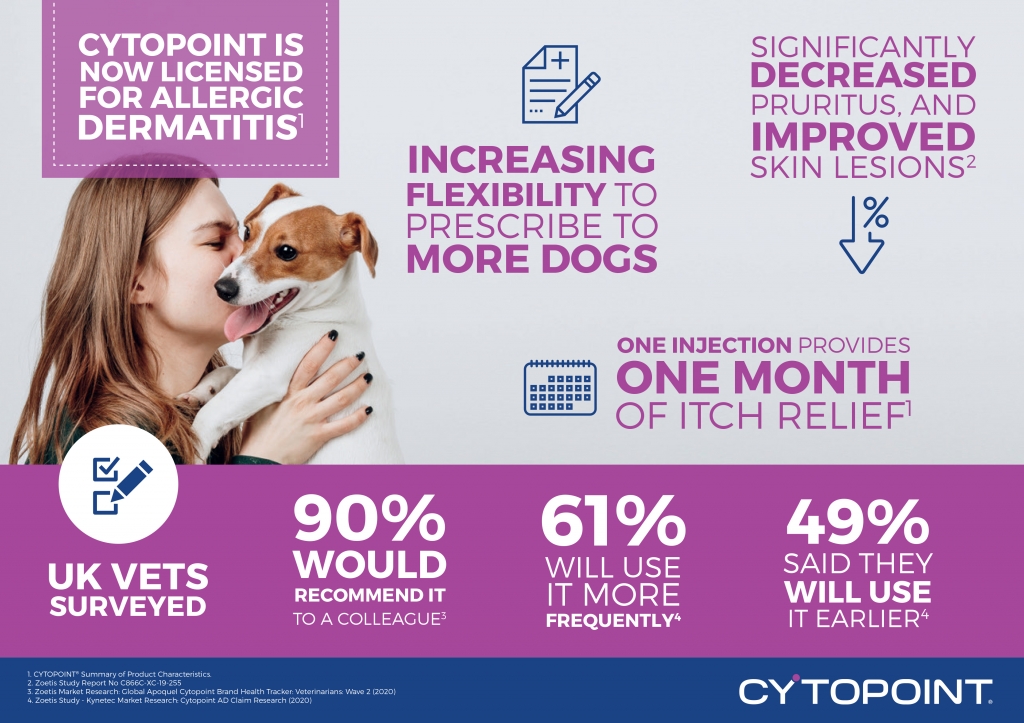
December 3, 2020 – The licence for Zoetis’ canine dermatitis product, CYTOPOINT® (lokivetmab), has been extended to include the treatment of pruritus associated with allergic dermatitis, in addition to the original claim of clinical manifestations of atopic dermatitis.[i]
CYTOPOINT is an injectable monoclonal antibody therapy for dogs that targets and neutralises the cytokine IL-31, a key mediator of allergic itch.[ii] Veterinarians are now able to offer the rapid and long-lasting itch relief seen with CYTOPOINT to more dogs suffering from pruritic skin disease. This presents an opportunity to improve treatment compliance by removing the hassle of giving tablets every day.
The new claim was granted following additional research in the form of a randomised, double-masked, multi-centred, placebo-controlled study which looked at CYTOPOINT’s efficacy and safety for the treatment of pruritus associated with allergic dermatitis in client-owned dogs. The study showed that CYTOPOINT significantly decreased pruritus, and improved skin lesions. [iii]
Allison Henry, Product Manager Companion Animal Dermatology at Zoetis UK, says: “Allergic dermatitis is one of the most common skin conditions in dogs.[iv] The associated clinical signs – including scratching, hair loss and skin lesions – are disruptive for the dog, impacting their quality of life and that of its owner. Allergic skin disease is one of the most frequent reasons owners present their dog to a veterinarian.
“The licence extension demonstrates further innovation from Zoetis in the field of veterinary dermatology, and across the continuum of care, increasing the treatment options available to manage pruritus. APOQUEL® remains an ideal choice for short-term control due to its ability to provide rapid itch relief within four hours[v] for as little as one day.[vi] This enables pruritus to be tightly controlled during the diagnostic work up,[vii] as well as being used for longer term control where tablets are the preferred method of administration.”
The sustained duration of action of CYTOPOINT makes it better suited to long-term management of pruritus, particularly when administering tablets is a challenge. Other benefits, such as no restrictions on age or with comorbidities, coupled with the flexibility of the new claim, make it a useful treatment at an earlier stage for more unique cases, e.g. young dogs or those with comorbidities.
Since its launch three years ago, CYTOPOINT has become a go-to therapy for UK vets, with 90% reporting that they would be likely to recommend it to a colleague.[viii] The license extension provides additional flexibility for veterinarians to prescribe CYTOPOINT for dogs with allergic dermatitis without the need to specifically diagnose atopic dermatitis first. This additional claim means that 61% of veterinarians said they will use CYTOPOINT more frequently following the new claim and 49% said they will use it earlier.[ix]
For further information on the Cytopoint licence extension please contact your Zoetis Account Manager.
[i] CYTOPOINT Summary of Product Characteristics.
[ii] Gonzales AJ, et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013; 24(1): 48−53. doi:10.1111/j.1365-3164.2012.01098.x
[iii] Zoetis Study Report No C866C-XC-19-255.
[iv] Hillier, A, Griffin, CE. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Veterinary Immunology and Immunopathology 1999; 81: 147-151.
[v] Gadeyne C, et al. Efficacy of oclacitinib (APOQUEL®) compared with prednisolone for the control of pruritus and clinical signs associated with allergic dermatitis in client-owned dogs in Australia. Vet Dermatol. 2014; 25(6): 512-8, e86. doi:10.1111/vde.12166.
[vi] APOQUEL® Summary of Product Characteristics.
[vii] Clear V, et al. Investigation of the effects of 30-day administration of oclacitinib (APOQUEL) on intradermal and allergen-specific IgE serology testing in atopic dogs. NAVDF proc, Nashville, TN. 2015; p 32.
[viii] Zoetis Market Research: Global APOQUEL CYTOPOINT Brand Health Tracker: Veterinarians: Wave 2 (2020)
[ix] Zoetis Study - Kynetec Market Research: CYTOPOINT AD Claim Research (2020).
More from Zoetis
- 93% of horse owners would vaccinate for EHV if their vet recommended it
- Zoetis calls vets to help test groundbreaking equine health-related quality of life (HRQL) assessment tool
- Vets encouraged to engage with their farmers on the importance of orf vaccination
- Zoetis Hosts Free Webinar to Share Latest Knowledge of Lungworm
- Vets Urge Action as Dog Arthritis Misconceptions Persist

 5 years ago
5 years ago  3328 views
3328 views
